








Today has 35 numerology, and the 35th prime = 149


Today is 11 weeks 6 days after the anniversary of his disappearance:

Today he would have been 61 years 10 weeks 1 day old:


Today is 42 weeks 2 days before his death anniversary:


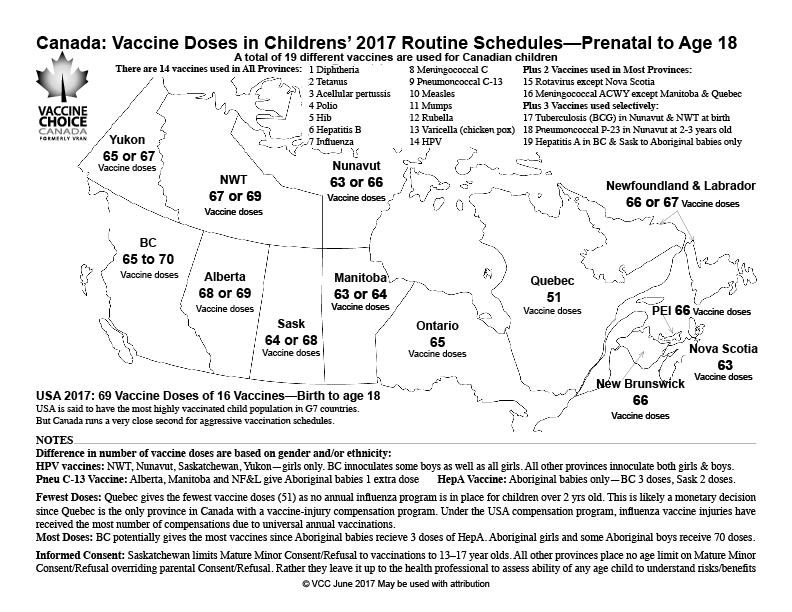
The “CDC Vaccine Schedule” = 72 consists of 72 doses of 16 vaccines to children all the way from day 1 (from birth) to 18 years of age to any given child in America. This practice of medicine is “Epidemiology” = 72:











His study saw the connection between the gut & autism, and noticed that the MMR COULD be involved. Decades later multiple studies confirm this.


Bullshit:




Here is a link which conatins 89 peer reviewed papers connecting autism and mercury:
https://childrenshealthdefense.org/wp-content/uploads/autism-mercury-abstracts-5.8.19.pdf





Link to 100 papers about Aluminum / Autism / Vaccines:
https://drive.google.com/file/d/0B3mMkPwF1DUPN3lnWXdyY0otVGM/view
































So that is the entire article from Scientific American. Before we continue here are just some small graphs that I’m going to save here for future reference:


Okay let’s look at this vaccine:

This leads to this paper published in 2016:




The presentation of this graph is that it will instill the idea that these mild reactions to the vaccine taper off to 0 in due time, but no evidence of this is provided.



So it is 100% effective, but they this isn’t a safety study. Now that they know there is a “100% efficacy rate,” where is the Vaccinated Vs. Vaccinated study, with a minimum of 3-4 year follow-up to see long term adverse events? This study only followed for 84 days. This was published online 3 years ago.
They have been using an unlicensed version already with no safety study, and will now transition to the licensed version.
Anaphylaxis and febrile reaction were causally related to the vaccine.
48% of serious adverse events were the Ebola virus disease ??
—
There are 3 major dates to be studied in within this vaccines lifespan to date:
12/22/2016 – Published online: Efficacy and eff ectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: fi nal results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffi t!)
11/11/19 – European commission accepts the vaccine.
12/20/19 – FDA licenses the vaccine.
https://www.statista.com/statistics/257364/top-lobbying-industries-in-the-us/






If we round up, then Hospitals/Nursing Homes = 101 million spent:


